Arrhenius acids
The Swedish chemist Svante Arrhenius attributed the properties of acidity to hydrogen in 1884. An Arrhenius acid is a substance that increases the concentration of the hydronium ion, H3O+, when dissolved in water. This definition stems from the equilibrium dissociation of water into hydronium and hydroxide (OH−) ions:- H2O(l) + H2O(l)
 H3O+(aq) + OH−(aq)
H3O+(aq) + OH−(aq)
In pure water the majority of molecules exist as H2O, but a small number of molecules are constantly dissociating and re-associating. Pure water is neutral with respect to acidity or basicity because the concentration of hydroxide ions is always equal to the concentration of hydronium ions. An Arrhenius base is a molecule which increases the concentration of the hydroxide ion when dissolved in water. Note that chemists often write H+(aq) and refer to the hydrogen ion when describing acid-base reactions but the free hydrogen nucleus, a proton, does not exist alone in water, it exists as the hydronium ion, H3O+.
Lewis acids
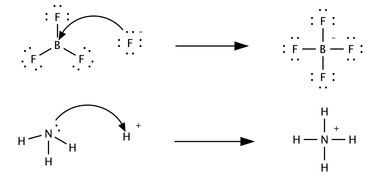
A third concept was proposed in 1923 by Gilbert N. Lewis which includes reactions with acid-base characteristics that do not involve a proton transfer. A Lewis acid is a species that accepts a pair of electrons from another species; in other words, it is an electron pair acceptor. Brønsted acid-base reactions are proton transfer reactions while Lewis acid-base reactions are electron pair transfers. All Brønsted acids are also Lewis acids, but not all Lewis acids are Brønsted acids. Contrast the following reactions which could be described in terms of acid-base chemistry.In the first reaction a fluoride ion, F−, gives up an electron pair to boron trifluoride to form the product tetrafluoroborate. Fluoride "loses" a pair of valence electrons because the electrons shared in the B—F bond are located in the region of space between the two atomic nuclei and are therefore more distant from the fluoride nucleus than they are in the lone fluoride ion. BF3 is a Lewis acid because it accepts the electron pair from fluoride. This reaction cannot be described in terms of Brønsted theory because there is no proton transfer. The second reaction can be described using either theory. A proton is transferred from an unspecified Brønsted acid to ammonia, a Brønsted base; alternatively, ammonia acts as a Lewis base and transfers a lone pair of electrons to form a bond with a hydrogen ion. The species that gains the electron pair is the Lewis acid; for example, the oxygen atom in H3O+ gains a pair of electrons when one of the H—O bonds is broken and the electrons shared in the bond become localized on oxygen. Depending on the context, a Lewis acid may also be described as an oxidizer or an electrophile.
The Brønsted-Lowry definition is the most widely used definition; unless otherwise specified acid-base reactions are assumed to involve the transfer of a proton (H+) from an acid to a base.