Greenhouse gas intensity and land-use change
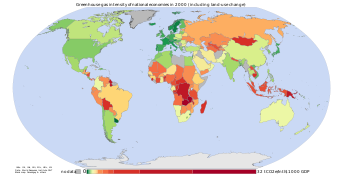
The figure opposite is based on data from the World Resources Institute, and shows a measurement of GHG emissions for the year 2000 according to greenhouse gas intensity and land-use change. Herzog et al. (2006, p. 3) defined greenhouse gas intensity as GHG emissions divided by economic output. GHG intensities are subject to uncertainty over whether they are calculated using market exchange rates (MER) or purchasing power parity (PPP) (Banuri et al., 1996, p. 96). Calculations based on MER suggest large differences in intensities between developed and developing countries, whereas calculations based on PPP show smaller differences.
Land-use change, e.g., the clearing of forests for agricultural use, can affect the concentration of GHGs in the atmosphere by altering how much carbon flows out of the atmosphere into carbon sinks. Accounting for land-use change can be understood as an attempt to measure “net” emissions, i.e., gross emissions from all GHG sources minus the removal of emissions from the atmosphere by carbon sinks (Banuri et al., 1996, pp. 92–93).
There are substantial uncertainties in the measurement of net carbon emissions. Additionally, there is controversy over how carbon sinks should be allocated between different regions and over time (Banuri et al., 1996, p. 93). For instance, concentrating on more recent changes in carbon sinks is likely to favour those regions that have deforested earlier, e.g., Europe.
Cumulative and historical emissions
| Region | Industrial CO2 | Total CO2 |
|---|---|---|
| OECD North America | 33.2 | 29.7 |
| OECD Europe | 26.1 | 16.6 |
| Former USSR | 14.1 | 12.5 |
| China | 5.5 | 6.0 |
| Eastern Europe | 5.5 | 4.8 |
The table above is based on Banuri et al. (1996, p. 94). Overall, developed countries accounted for 83.8% of industrial CO2 emissions over this time period, and 67.8% of total CO2 emissions. Developing countries accounted for industrial CO2 emissions of 16.2% over this time period, and 32.2% of total CO2 emissions. The estimate of total CO2 emissions includes biotic carbon emissions, mainly from deforestation. Banuri et al. (1996, p. 94) calculated per capita cumulative emissions based on then-current population. The ratio in per capita emissions between industrialized countires and developing countries was estimated to be more than 10 to 1.
Including biotic emissions brings about the same controversy mentioned earlier regarding carbon sinks and land-use change (Banuri et al., 1996, pp. 93–94). The actual calculation of net emissions is very complex, and is affected by how carbon sinks are allocated between regions (an equity consideration), and the dynamics of the climate system.
The International Energy Agency (IEA, 2007, p. 201) compared cumulative energy-related CO2 emissions for several countries and regions. Over the time period 1900-2005, the US accounted for 30% of total cumulative emissions; the EU, 23%; China, 8%; Japan, 4%; and India, 2%. The rest of the world accounted for 33% of global, cumulative, energy-related CO2 emissions.
Changes since a particular base year
In total, Annex I Parties managed a cut of 3.3% in GHG emissions between 1990 and 2004 (UNFCCC, 2007, p. 11). Annex I Parties are those countries listed in Annex I of the UNFCCC, and are the industrialized countries. For non-Annex I Parties, emissions in several large developing countries and fast growing economies (China, India, Thailand, Indonesia, Egypt, and Iran) GHG emissions have increased rapidly over this period (PBL, 2009).The sharp acceleration in CO2 emissions since 2000 to more than a 3% increase per year (more than 2 ppm per year) from 1.1% per year during the 1990s is attributable to the lapse of formerly declining trends in carbon intensity of both developing and developed nations. China was responsible for most of global growth in emissions during this period. Localised plummeting emissions associated with the collapse of the Soviet Union have been followed by slow emissions growth in this region due to more efficient energy use, made necessary by the increasing proportion of it that is exported. In comparison, methane has not increased appreciably, and N2O by 0.25% y−1.
Annual and per capita emissions
At the present time, total annual emissions of GHGs are rising (Rogner et al., 2007). Between the period 1970 to 2004, emissions increased at an average rate of 1.6% per year, with CO2 emissions from the use of fossil fuels growing at a rate of 1.9% per year.Today, the stock of carbon in the atmosphere increases by more than 3 million tonnes per annum (0.04%) compared with the existing stock. This increase is the result of human activities by burning fossil fuels, deforestation and forest degradation in tropical and boreal regions.
Per capita emissions in the industrialized countries are typically as much as ten times the average in developing countries (Grubb, 2003, p. 144). Due to China's fast economic development, its per capita emissions are quickly approaching the levels of those in the Annex I group of the Kyoto Protocol (PBL, 2009). Other countries with fast growing emissions are South Korea, Iran, and Australia. On the other hand, per capita emissions of the EU-15 and the USA are gradually decreasing over time. Emissions in Russia and the Ukraine have decreased fastest since 1990 due to economic restructuring in these countries (Carbon Trust, 2009, p. 24).
Energy statistics for fast growing economies are less accurate than those for the industrialized countries. For China's annual emissions in 2008, PBL (2008) estimated an uncertainty range of about 10%.
Top emitters
In 2005, the world's top-20 emitters comprised 80% of total GHG emissions (PBL, 2010. See notes for the following table). Tabulated below are the top-5 emitters for the year 2005 (MNP, 2007). The second column is the country's or region's share of the global total of annual emissions. The third column is the country's or region's average annual per capita emissions, in tonnes of GHG per head of population:| Country or region | % of global total annual emissions | Tonnes of GHG per capita |
|---|---|---|
| United Statesa | 16 % | 24.1 |
| Indonesiac | 6 % | 12.9 |
| European Union-27a | 11 % | 10.6 |
| Chinab | 17 % | 5.8 |
| India | 5 % | 2.1 |
Table footnotes:
- These values are for the GHG emissions from fossil fuel use and cement production. Calculations are for carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O) and gases containing fluorine (the F-gases HFCs, PFCs and SF6).
- These estimates are subject to large uncertainties regarding CO2 emissions from deforestation; and the per country emissions of other GHGs (e.g., methane). There are also other large uncertainties which mean that small differences between countries are not significant. CO2 emissions from the decay of remaining biomass after biomass burning/deforestation are not included.
- a Industrialised countries: official country data reported to UNFCCC.
- b Excluding underground fires.
Embedded emissions
One way of attributing greenhouse gas (GHG) emissions is to measure the embedded emissions (also referred to as "embodied emissions") of goods that are being consumed. Emissions are usually measured according to production, rather than consumption (Helm et al., 2007, p. 3). Under a production-based accounting of emissions, embedded emissions on imported goods are attributed to the exporting, rather than the importing, country. Under a consumption-based accounting of emissions, embedded emissions on imported goods are attributed to the importing country, rather than the exporting, country.Davis and Caldeira (2010, p. 4) found that a substantial proportion of CO2 emissions are traded internationally. The net effect of trade was to export emissions from China and other emerging markets to consumers in the US, Japan, and Western Europe. Based on annual emissions data from the year 2004, and on a per-capita consumption basis, the top-5 emitting countries were found to be (in tCO2 per person, per year): Luxembourg (34.7), the US (22.0), Singapore (20.2), Australia (16.7), and Canada (16.6) (Davis and Caldeira, 2010, p. 5).
Effect of policy
Rogner et al. (2007) assessed the effectiveness of policies to reduce emissions (mitigation of climate change). They concluded that mitigation policies undertaken by UNFCCC Parties were inadequate to reverse the trend of increasing GHG emissions. The impacts of population growth, economic development, technological investment, and consumption had overwhelmed improvements in energy intensities and efforts to decarbonize (energy intensity is a country's total primary energy supply (TPES) per unit of GDP (Rogner et al., 2007). TPES is a measure of commercial energy consumption (World Bank, 2010, p. 371)).Projections
Based on then-current energy policies, Rogner et al. (2007) projected that energy-related CO2 emissions in 2030 would be 40-110% higher than in 2000. Two-thirds of this increase was projected to come from non-Annex I countries. Per capita emissions in Annex I countries were still projected to remain substantially higher than per capita emissions in non-Annex I countries. Projections consistently showed a 25-90% increase in the Kyoto gases (carbon dioxide, methane, nitrous oxide, sulphur hexafluoride) compared to 2000.
IEA (2007, p. 199) estimated future cumulative energy-related CO2 emissions for several countries. Their reference scenario projected cumulative energy-related CO2 emissions between the years 1900 and 2030. In this scenario, China’s share of cumulative emissions rises to 16%, approaching that of the United States (25%) and the European Union (18%). India’s cumulative emissions (4%) approach those of Japan (4%).
Relative CO2 emission from various fuelsOne liter of gasoline, when used as a fuel, produces 2.32 kg (1.3 cubic meters) of carbon dioxide, a greenhouse gas. One US gallon produces 19.4 lb (172.65 cubic feet)
Fuel name | CO2 emitted (lbs/106 Btu)  | CO2 emitted (g/106 J)  |
|---|---|---|
| Natural gas | 117 | 50.30 |
| Liquefied petroleum gas | 139 | 59.76 |
| Propane | 139 | 59.76 |
| Aviation gasoline | 153 | 65.78 |
| Automobile gasoline | 156 | 67.07 |
| Kerosene | 159 | 68.36 |
| Fuel oil | 161 | 69.22 |
| Tires/tire derived fuel | 189 | 81.26 |
| Wood and wood waste | 195 | 83.83 |
| Coal (bituminous) | 205 | 88.13 |
| Coal (subbituminous) | 213 | 91.57 |
| Coal (lignite) | 215 | 92.43 |
| Petroleum coke | 225 | 96.73 |
| Coal (anthracite) | 227 | 97.59 |
Removal from the atmosphere and global warming potential
Natural processes
Greenhouse gases can be removed from the atmosphere by various processes, as a consequence of:
- a physical change (condensation and precipitation remove water vapor from the atmosphere).
- a chemical reactions within the atmosphere. For example, methane is oxidized by reaction with naturally occurring hydroxyl radical, OH· and degraded to CO2 and water vapor (CO2 from the oxidation of methane is not included in the methane Global warming potential). Other chemical reactions include solution and solid phase chemistry occurring in atmospheric aerosols.
- a physical exchange between the atmosphere and the other compartments of the planet. An example is the mixing of atmospheric gases into the oceans.
- a chemical change at the interface between the atmosphere and the other compartments of the planet. This is the case for CO2, which is reduced by photosynthesis of plants, and which, after dissolving in the oceans, reacts to form carbonic acid and bicarbonate and carbonate ions (see ocean acidification).
- a photochemical change. Halocarbons are dissociated by UV light releasing Cl· and F· as free radicals in the stratosphere with harmful effects on ozone (halocarbons are generally too stable to disappear by chemical reaction in the atmosphere).
Aside from water vapor, which has a residence time of about nine days, major greenhouse gases are well-mixed, and take many years to leave the atmosphere. Although it is not easy to know with precision how long it takes greenhouse gases to leave the atmosphere, there are estimates for the principal greenhouse gases. Jacob (1999) defines the lifetime τ of an atmospheric species X in a one-box model as the average time that a molecule of X remains in the box. Mathematically τ can be defined as the ratio of the mass m (in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box (Fout), chemical loss of X (L), and deposition of X (D) (all in kg/sec):
 The atmospheric lifetime of a species therefore measures the time required to restore equilibrium following an increase in its concentration in the atmosphere. Individual atoms or molecules may be lost or deposited to sinks such as the soil, the oceans and other waters, or vegetation and other biological systems, reducing the excess to background concentrations. The average time taken to achieve this is the mean lifetime. The atmospheric lifetime of CO2 is often incorrectly stated to be only a few years because that is the average time for any CO2 molecule to stay in the atmosphere before being removed by mixing into the ocean, photosynthesis, or other processes. However, this ignores the balancing fluxes of CO2 into the atmosphere from the other reservoirs. It is the net concentration changes of the various greenhouse gases by all sources and sinks that determines atmospheric lifetime, not just the removal processes.
The atmospheric lifetime of a species therefore measures the time required to restore equilibrium following an increase in its concentration in the atmosphere. Individual atoms or molecules may be lost or deposited to sinks such as the soil, the oceans and other waters, or vegetation and other biological systems, reducing the excess to background concentrations. The average time taken to achieve this is the mean lifetime. The atmospheric lifetime of CO2 is often incorrectly stated to be only a few years because that is the average time for any CO2 molecule to stay in the atmosphere before being removed by mixing into the ocean, photosynthesis, or other processes. However, this ignores the balancing fluxes of CO2 into the atmosphere from the other reservoirs. It is the net concentration changes of the various greenhouse gases by all sources and sinks that determines atmospheric lifetime, not just the removal processes.Global warming potential
The global warming potential (GWP) depends on both the efficiency of the molecule as a greenhouse gas and its atmospheric lifetime. GWP is measured relative to the same mass of CO2 and evaluated for a specific timescale. Thus, if a gas has a high radiative forcing but also a short lifetime, it will have a large GWP on a 20 year scale but a small one on a 100 year scale. Conversely, if a molecule has a longer atmospheric lifetime than CO2 its GWP will increase with the timescale considered.
Carbon dioxide has a variable atmospheric lifetime, and cannot be specified precisely. Recent work indicates that recovery from a large input of atmospheric CO2 from burning fossil fuels will result in an effective lifetime of tens of thousands of years. Carbon dioxide is defined to have a GWP of 1 over all time periods.
Methane has an atmospheric lifetime of 12 ± 3 years and a GWP of 72 over 20 years, 25 over 100 years and 7.6 over 500 years. The decrease in GWP at longer times is because methane is degraded to water and CO2 through chemical reactions in the atmosphere.
Examples of the atmospheric lifetime and GWP relative to CO2 for several greenhouse gases are given in the following table:
| Gas name | Chemical formula | Lifetime (years) | Global warming potential (GWP) for given time horizon | ||
|---|---|---|---|---|---|
| 20-yr | 100-yr | 500-yr | |||
| Carbon dioxide | CO2 | See above | 1 | 1 | 1 |
| Methane | CH4 | 12 | 72 | 25 | 7.6 |
| Nitrous oxide | N2O | 114 | 289 | 298 | 153 |
| CFC-12 | CCl2F2 | 100 | 11 000 | 10 900 | 5 200 |
| HCFC-22 | CHClF2 | 12 | 5 160 | 1 810 | 549 |
| Tetrafluoromethane | CF4 | 50 000 | 5 210 | 7 390 | 11 200 |
| Hexafluoroethane | C2F6 | 10 000 | 8 630 | 12 200 | 18 200 |
| Sulphur hexafluoride | SF6 | 3 200 | 16 300 | 22 800 | 32 600 |
| Nitrogen trifluoride | NF3 | 740 | 12 300 | 17 200 | 20 700 |
Airborne fraction
Airborne fraction (AF) is the proportion of an emission (e.g. CO2) remaining in the atmosphere after a specified time. Canadell (2007) define the annual AF as the ratio of the atmospheric CO2 increase in a given year to that year’s total emissions, and calculate that of the average 9.1 PgC y−1 of total anthropogenic emissions from 2000 to 2006, the AF was 0.45. For CO2 the AF over the last 50 years (1956–2006) has been increasing at 0.25 ± 0.21%/year.
Negative emissions
Related effects
Carbon monoxide has an indirect radiative effect by elevating concentrations of methane and tropospheric ozone through scavenging of atmospheric constituents (e.g., the hydroxyl radical, OH) that would otherwise destroy them. Carbon monoxide is created when carbon-containing fuels are burned incompletely. Through natural processes in the atmosphere, it is eventually oxidized to carbon dioxide. Carbon monoxide has an atmospheric lifetime of only a few months and as a consequence is spatially more variable than longer-lived gases.
Another potentially important indirect effect comes from methane, which in addition to its direct radiative impact also contributes to ozone formation. Shindell et al. (2005) argue that the contribution to climate change from methane is at least double previous estimates as a result of this effect.<
 A greenhouse gas (sometimes abbreviated GHG) is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone. In the Solar System, the atmospheres of Venus, Mars, and Titan also contain gases that cause greenhouse effects. Greenhouse gases greatly affect the temperature of the Earth; without them, Earth's surface would be on average about 33 °C (59 °F) colder than at present.
A greenhouse gas (sometimes abbreviated GHG) is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone. In the Solar System, the atmospheres of Venus, Mars, and Titan also contain gases that cause greenhouse effects. Greenhouse gases greatly affect the temperature of the Earth; without them, Earth's surface would be on average about 33 °C (59 °F) colder than at present.





 World Environment Day (WED) is a day that stimulates awareness of the environment and enhances political attention and public action. It is on 5 June. It was the day that United Nations Conference on the Human Environment began. The United Nations Conference on the Human Environment was from 5–16 June 1972. It was established by the United Nations General Assembly in 1972. The first World Environment Day was on 1973. World Environment Day is hosted every year by a different city with a different theme and is commemorated with an international exposition in the week of 5 June. World Environment Day is in summer in the Northern Hemisphere and winter in the Southern Hemisphere.
World Environment Day (WED) is a day that stimulates awareness of the environment and enhances political attention and public action. It is on 5 June. It was the day that United Nations Conference on the Human Environment began. The United Nations Conference on the Human Environment was from 5–16 June 1972. It was established by the United Nations General Assembly in 1972. The first World Environment Day was on 1973. World Environment Day is hosted every year by a different city with a different theme and is commemorated with an international exposition in the week of 5 June. World Environment Day is in summer in the Northern Hemisphere and winter in the Southern Hemisphere.